DIOSMIN
Identification
Ingredient |
Diosmin |
Structure:
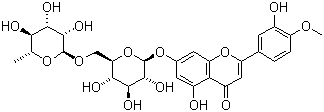
|
Synonyms |
3',5,7-trihydroxy-4'-methoxyflavone-7-rutinoside |
Molecular Formula |
C28H32O15 |
Molecular Weight |
608.54 |
CAS no. |
520-27-4 |
EINECS |
208-289-7 |
Description
Diosmin is greyish-yellow or light yellow hygroscopic powder,practically insoluble in water, soluble in dimethyl sulphoxide, practically insoluble in alcohol. It dissolves in dilute solutions of alkali hydroxides. Melting point is 275-277 °C when purity is 95% tested by HPLC.
Scientific support
Diosmin was first isolated in 1925 from Scrophularia nodosa, and first introduced as a therapeutic agent in 1969. Diosmin is considered to be a vascular-protecting agent used to treat chronic venous insufficiency, hemorrhoids, lymphedema, and varicose veins. As a flavonoid, diosmin also exhibits anti-inflammatory, free-radical scavenging, and antimutagenic properties.
Diosmin differs molecularly from hesperidin by the presence of a double bond between two carbon atoms in diosmin's central carbon ring. Diosmin can be manufactured by extracting hesperidin from citrus rinds, followed by conversion of hesperidin to diosmin. Diosmin has been used for more than 30 years as a phlebotonic and vascular-protecting agent, and has recently begun to be investigated for other therapeutic purposes, including cancer, premenstrual syndrome, colitis, and diabetes.
Biochemistry and Pharmacokinetics
Flavonoids are a large group of plant pigments sharing the same basic chemical structure; i.e., a three-ringed molecule with hydroxyl (OH) groups attached. Diosmin occurs naturally as a glycoside, meaning it has a sugar molecule attached to its three-ringed flavonoid structure.
Pharmacokinetic investigations have shown diosmin is rapidly transformed by intestinal flora to its aglycone form, diosmetin. Diosmetin is absorbed and rapidly distributed throughout the body with a plasma half-life of 26-43 hours. Diosmetin is degraded to phenolic acids or their glycine-conjugated derivatives and eliminated through the urine. Diosmin or diosmetin not absorbed is eliminated in the feces.
Mechanisms of Action
Diosmin's mechanisms of action include improvement of venous tone, increased lymphatic drainage, protection of capillary bed microcirculation, inhibition of inflammatory reactions, and reduced capillary permeability. Certain flavonoids, including diosmin, are potent inhibitors of prostaglandin E2 (PGE2) and thromboxane A2 (TxA2) as well as being inhibitors of leukocyte activation, migration, and adhesion. Diosmin causes a significant decrease in plasma levels of endothelial adhesion molecules and reduces neutrophil activation, thus providing protection against microcirculatory damage.
Clinical Indications
Varicose Veins/Chronic Venous Insufficiency
Chronic venous insufficiency is characterized by pain, leg heaviness, a sensation of swelling, and cramps, and is correlated with varicose veins. A multicenter international trial, carried out in 23 countries over two years, in which 5,052 symptomatic patients were enrolled, evaluated the efficacy of flavonoids in the treatment of chronic venous insufficiency. Patients were treated with 450 mg diosmin and 50 mg hesperidin daily for six months. Continuous clinical improvement was found throughout the study, as well as improvements in quality of life scores for participants.
Diosmin-containing flavonoid mixtures have also been effective in treating severe stages of chronic venous insufficiency, including venous ulceration and delayed healing. In a randomized multicenter trial, 900 mg diosmin and 100 mg hesperidin plus standard venous ulcer management was compared with standard venous ulcer management alone. Standard ulcer management included cleaning, compression therapy, and skin care of the adjacent skin. Forty-seven percent of patients in the treatment group compared to 28 percent in the standard management group experienced complete healing of ulcers less than 10 cm in diameter.
Hemorrhoids
Several large clinical trials have demonstrated diosmin to be effective in the treatment of acute and chronic symptoms of hemorrhoids. A double-blind, placebo-controlled study of 120 patients showed improvement of pain, pruritis, discharge, edema, erythema, and bleeding on examination. The treatment group was given a flavonoid mixture (90% diosmin and 10% hesperidin) at a dose of two 500-mg tablets daily for two months.
The use of diosmin in the treatment of hemorrhoids associated with pregnancy did not adversely affect pregnancy, fetal development, birth weight, infant growth, or infant feeding. Pregnant women suffering from acute hemorrhoids were treated eight weeks before delivery and four weeks after delivery. More than half of the women participating in the study reported relief from symptoms by the fourth day. Diosmin is non-mutagenic and does not have any significant effect on reproductive function.
Lymphedema
Diosmin acts on the lymphatic system by increasing lymph flow and lymph oncotic pressure. A flavonoid mixture containing diosmin was used to treat upper limb lymphedema secondary to conventional therapy for breast cancer. Results showed improvement of symptoms and limb volume; the mean decrease in volume of the swollen limb reached 6.8 percent. In addition, lymphatic functional parameters assessed with scintigraphy were significantly improved. Animal studies of high-protein lymphedema, such as in burns and lung contusions, showed significant improvement with diosmin.
Diabetes
Diosmin has been shown to improve factors associated with diabetic complications. Blood parameters of glycation and oxidative stress were measured in type 1 diabetic patients before and after intervention with a diosmin-containing flavonoid mixture. A decrease in hemoglobin Ale was accompanied by an increase in glutathione peroxidase, demonstrating long-term decreased blood glucose levels and increased antioxidant activity.
Diosmin can normalize capillary filtration rate and prevent ischemia in diabetics. Rheological studies of type 1 diabetics show diosmin can facilitate hemorheological improvements due to decreased RBC aggregation, which decreases blood flow resistance, resulting in reduction of both stasis and ischemia.
Cancer
Diosmin has been investigated in a number of animal models and human cancer cell lines, and has been found to be chemopreventive and antiproliferative. More clinically oriented research in this area is warranted to determine effective dosages and protocols.
Other Clinical Indications
Studies have also investigated the use of diosmin for stasis dermatitis, wound healing, premenstrual syndrome, mastodynia, dermatofibrosclerosis, viral infections, and colitis. More clinically oriented research is indicated.
Drug-Nutrient Interactions
Diosmin can cause a decrease in RBC aggregation and blood viscosity.There are no documented cases of adverse interactions between diosmin and prescription medications, but caution should be taken when combining diosmin with aspirin or other blood-thinning medications.
Data suggest that diosmin has an inhibitory effect on cytochrome P450-mediated metabolism in healthy volunteers, which may alter the pharmacokinetics of drugs taken concomitantly. Patients given metronidazole after nine days of pretreatment with 450 mg diosmin demonstrated changes in serum concentrations of metronidazole, as well as changes in urinary concentrations of metronidazole and its metabolites compared to controls.
Side Effects and Toxicity
In animal studies, a flavonoid mixture containing 90-percent diosmin and 10-percent hesperidin had an L[D.sub.50] of more than 3g/kg. In addition, animal studies have shown the absence of acute, subacute, or chronic toxicity after repeated oral dosing for 13 and 26 weeks using a dose representing 35 times the recommended daily dose.
Diosmin is considered to have no mutagenic activity, embryo toxicity, nor any significant effect on reproductive function. Transplacental migration and passage into breast milk are mini-real.
Dosage
The standard dose of diosmin is 500 mg twice daily. For acute dosing, a loading dose of 1,000 mg three times daily for four days is recommended, followed by 1,000 mg twice daily for three days, and a maintenance dose of 500 mg twice daily for two months.
Application
Diosmin is largely used in EU as a medicine in combination with Hesperidine. The formulation of Dismin 90%+Hesperidin 10% branded Alvenor by famous Servier has an annual sales of millions of dollars in EU. But FDA does not approve it as a medicine but a dietary supplement. Recently a new formulation of Diosmin 95%+Hesperidin 5% has been also approved by EU countries and FDA.
Nowadays more companies in other countries also launched similar products as the same proportion of Diosmin and Hesperidine, whatever it is used as a medicine or dietary supplement, Diosmin has been well acknowledged to be a vascular-protecting agent used to treat chronic venous insufficiency, hemorrhoids, lymphedema, and varicose veins.
Availability
Commercial Diosmin is obtained by chemical synthesis from hesperidin (extracted from immature citrus fruit).

Dried immature citrus fruit
Warning:
Above information is only knowledge,not an instruction of usage for this product. The owner of this webiste should not be responsible for any damage because of misuse of the product. |



